Current Size: 100%
You are here
Home » Home » ResearchPalmitoylethanolamide Stimulates Phagocytosis by Microglial Cells without Inducing an Inflammatory Reaction
Summary: Palmitoylethanolamide (PEA), an endogenous lipid, increased phagocytosis of bacteria by microglial cells in vitro without a measurable proinflammatory effect. This allows the immune system to kill pathogens without harming nerve cells. Palmitoylethanolamide has been extensively tested clinically without observed severe side effects.
Palmitoylethanolamide (PEA) is a small endogenous lipid (molecular mass 299.5 g/mol) which is widely present in cells including microglia (Muccioli and Stella, 2008), tissues and body fluids. It has analgesic, anticonvulsant, neuroprotective, antipyretic and anti-inflammatory properties. Its actions depend mainly on the peroxisome proliferator-activated receptor (PPAR)α, but it also is a ligand of the transient receptor potential vanilloid-1 (TRPV1) and the orphan G-protein coupled receptor GPR55 (De Petrocellis et al., 2001; LoVerme et al., 2005, 2006; Ryberg et al., 2007; Esposito and Cuzzocrea, 2013). In a murine Theiler’s virus model of chronic MS, treatment with PEA (5 mg/kg) between days 60 and 70 post-infection resulted in a strong improvement of motor deficits caused by a reduction of microglial activation observed in untreated mice (Loría et al., 2008).
In spite of its anti-inflammatory properties, 30 min pre-treatment with PEA stimulated phagocytosis of S. pneumoniae (EC50 5.9 nM) and E. coli (EC50 23 nM) by microglial cells in vitro. It was not toxic to microglial cells up to a concentration of 1000 nM. Unlike pre-stimulation with TLR and NOD2 agonists, the PEA-mediated increase of microglial bacterial uptake was not accompanied by a release of pro-inflammatory cyto-/chemokines [TNFα, IL-6, and CXCL1 (KC)], avoiding the risk of concomitant neuronal injury (Redlich et al., 2012). Preliminary data suggest that PEA also decreases the susceptibility of the brain to intracerebral injection of bacteria (Sandra Redlich, unpublished data). From 1969 to 1979, PEA was tested under the brand name ImpulsinR (SPOFA United Pharmaceutical Works, Prague, Czechoslovakia) in prophylactic and therapeutic clinical trials (five in adults, one in children): it reduced the incidence and severity of acute respiratory infections and influenza (Kahlich et al., 1979; Keppel Hesselink et al., 2013). More than 3600 patients received PEA at daily doses from 600 to 1800 mg, and no severe adverse effects were reported (Kahlich et al., 1979; Keppel Hesselink et al., 2013).These properties illustrate that PEA is a true immunomodulator and not an immunosuppressant and make PEA a promising agent to enhance the resistance of the brain against infection without carrying the risk of inducing neuronal injury (Figure 6). This effect may be of clinical value both in preventing bacterial CNS infections in high-risk groups and in reducing the invasion of pathogens into brain tissue in manifest meningeal infections.
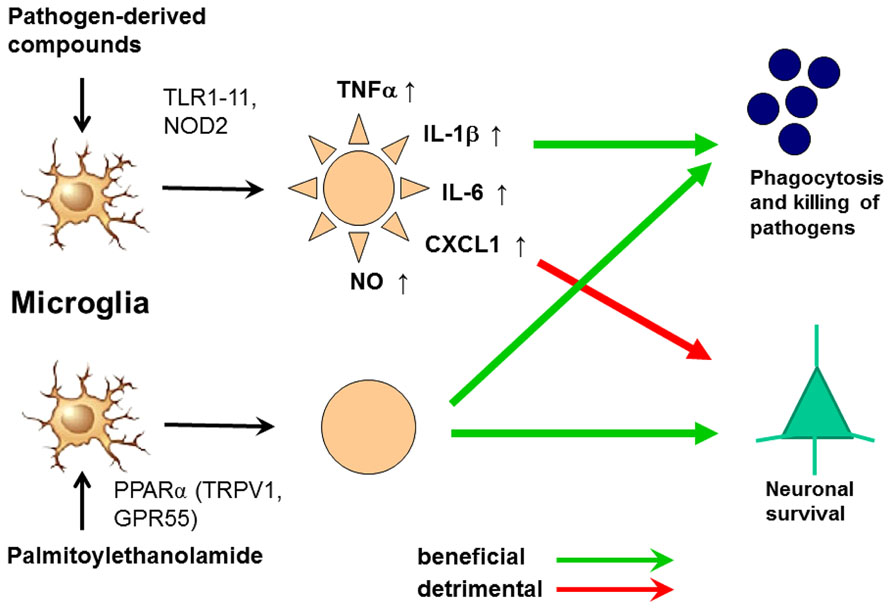
FIGURE 6. Activation of microglia by Toll-like receptor (TLR) agonists and by palmitoylethanolamide (PEA). The activation by both ways leads to an increase of phagocytosis and intracellular killing of pathogens. Stimulation of one or several TLR or nucleotide-binding oligomerization domain-containing protein 2 (NOD2) receptors causes the release of proinflammatory products from microglial cells causing neuronal injury in microglial-neuronal co-cultures and probably also in vivo. PEA also increases phagocytosis and intracellular killing of pathogens. To our knowledge, it does not release proinflammatory mediators. For this reason we hypothesize that it will not cause collateral neuronal injury. PEA probably acts via the peroxisome proliferator-activated receptor (PPAR)α, but also is a ligand of the transient receptor potential vanilloid-1 (TRPV1) and the orphan G-protein coupled receptor GPR55 (De Petrocellis et al., 2001; LoVerme et al., 2005, 2006; Ryberg et al., 2007; Esposito and Cuzzocrea, 2013).
Source:
Hypothesis & Theory ARTICLE
Front. Cell. Neurosci., 22 May 2014 | doi: 10.3389/fncel.2014.00138
Strategies to increase the activity of microglia as efficient protectors of the brain against infections
http://journal.frontiersin.org/Journal/10.3389/fncel.2014.00138/full

