Current Size: 100%
You are here
Home » Home » ResearchPalmitoylethanolamide Origin, History and Background
The chemical name of PeaPure is N-palmitoylethanolamide. (This is often abbreviated as PEA, although this can be confusing because PEA can refer to other substances.)
Palmitoylethanolamide is an natural (endogenous) fatty acid amide belonging to the family known as N-acylethanolamines (NAE's; or N-acylethanolamides), including 'cannabis-like' compounds such as N-arachidonoylethanolamide (anandamide, AEA).
Palmitoylethanolamide and AEA, have the potential to treat a range of human/ animal conditions involving abnormal inflammatory and/or immune responses and associated pain (see Lambert et al., 2002, for a review).
History and Scientific Literature Overview of Palmitoylethanolamide
Origin
Palmitoylethanolamide is a naturally occurring lipid found in many different cells of animal, marine and plant origin (see Lambert et al., 2002, for a review). In living organisms, palmitoylethanolamide synthesis is rapidly induced in response to cellular stressors, such as tissue damage or pathological insults, which are often accompanied by inflammation and pain (Darmani, et al., 2005). While the biological functions of palmitoylethanolamide in humans are not completely understood, it has been hypothesized that palmitoylethanolamide constitutes one of several natural anti-inflammatory and analgesic chemicals (Darmani, et al., 2005).
The crude lipid extracted from the freeze-dried flesh of Perna canaliculus using chloroform and methanol consists of a complex mixture of triglycerides, sterol esters, sterols, polar lipids, free fatty acids and their derivatives, such as NAEs (Sepe et al., 1998; Murphy et al., 2002; Murphy et al., 2003). Palmitoylethanolamide and N-stearoylethanolamide are the most abundant NAEs in blue mussel lipid extracts along with much smaller amounts of N-myristoyl (C14:0)-, N- oleoyl (C18:1)-, N-linoleoyl (C18:2)-, and N-arachidonoyl (C20:4; anandamide/ AEA)- ethanolamides. The nature and number of NAE's present in a crude or purified extract may have therapeutic implications since the prior art has revealed the existence of complex synergistic interactions between NAE's in whole animals and animal tissues. For example, data from animal studies indicates that some actions of palmitoylethanolamide may be mediated via AEA and possibly other NAEs. Furthermore, evidence of synergistic effects between NAEs have been reported (see below for details).
Chemistry
Palmitoylethanolamide is a saturated 16 carbon fatty acid ethanolamide (C16:0), with the structure:
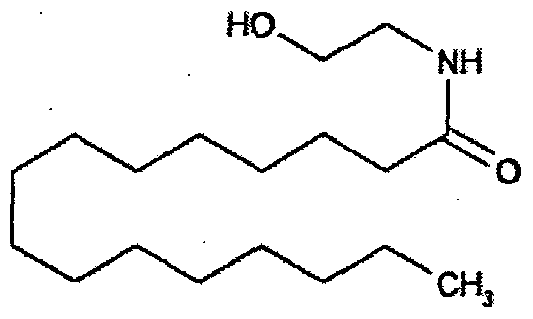
Historical Perspective
Interest in the anti-inflammatory properties of palmitoylethanolamide was first noted in the early 1950's by Coburn et al. (1954) who found that feeding guinea pigs a diet high in egg yolk protected them from experimental allergy. Subsequent studies isolated and purified palmitoylethanolamide from egg yolk and identified the anti-inflammatory properties in animals (see Lambert et al., 2002, for a review). Research interest in NAE's was revived in the 1990's following the discovery of another endogenous NAE, AEA, with similar properties to the active component of cannabis. The cloning of the cannabinoid receptors (designated CBi and CB2) and the generation of selective CB receptor ligands provided the tools to further accelerate research activity (Devane et al., 1992). Since then a substantial body of evidence gathered from animal and human studies has shown that palmitoylethanolamide has anti-inflammatory and analgesic properties when given via different routes of administration.
Pre-Clinical Studies
A number of studies have shown that synthetic palmitoylethanolamide has anti-inflammatory and analgesic properties in a range of animal models. Typically, an inflammatory substance, such as carrageenan, collagen or phorbol ester, is injected beneath the skin and the resulting pathological and behavioural changes are measured within hours (acute model) or several days (chronic model). To date, the vast majority of these studies have been conducted in acute animal models of inflammation (e.g., Aloe et al., 1993; Mazzari et al., 1996; Conti et al., 2002; Costa et al., 2002).
Clinical Trials
In comparison to animal studies, there have only been a small number of human clinical trials to investigate the anti-inflammatory effects of palmitoylethanolamide. During the early 1970's several trials were conducted in Czechoslovakia using an oral formulation given the trade name Impulsin™ (N-2-Hydroxyethyl palmitamide, SPOFA United Pharmaceutical Works). The first set of trials evaluated the efficacy of palmitoylethanolamide (3 times/ day/ 12 days) to reduce the incidence and severity of respiratory tract infections in 1345 adult volunteers (either young male soldiers or employees of the Skoda Car Co.; Masek et al., 1974). Results indicated that Impulsin™ helped to prevent viral infections when given before an infectious episode, but did not reduce the duration of infectious symptoms. A further series of similar trials conducted between 1973 and 1975, on a total of 1864 young male soldiers (same dose regime), confirmed that prophylactic Impulsin™ significantly reduced the incidence of acute respiratory infections in this population (Kahlich et al., 1979). The incidence of unwanted effects during the 12 week trial was particularly low (just a few percent; Kahlich et al., 1979). The apparent success of these trials led to the use of Impulsin™ in the former Czechoslovakia for acute respiratory disease. After several years on the market, the drug was withdrawn for unknown reasons which do not appear to be related to toxicity (see Lo Verme et al., 2005b, for a review). Two subsequent clinical trials have been initiated to investigate the efficacy of palmitoylethanolamide for chronic back pain (lumbosciatalgia) and multiple sclerosis (see Lambert et al., 2002, for a review).
A cream containing "structured natural lipids" with palmitoylethanolamide for topical administration has also been developed (Physiogel™ A.I., Stiefel Laboratories) and evaluated in two small-scale clinical trials. The first was an observational study in which 19 adult patients diagnosed with anal eczema were instructed to apply the cream to the affected area for between 6 and 63 days (Rohde & Ghyczy, 2003). After 4 weeks of the trial, 68% of patients reported a reduction in pain, burning sensation and itch, with 21% recording a worsening of symptoms. The cream was reportedly well tolerated by 95% patients. In the second trial, 21 adult patients with end-stage kidney failure and suffering from uremic itch, applied the cream twice daily for three weeks (Szepietowski et al., 2005). At the conclusion of the 3 week test period there was a statistically significant reduction in itch (completely absent in just under 40% patients) and 81% of patients reported an elimination of dryness in the affected area (xerosis). The cream was well tolerated by all patients with no adverse effects reported. These results provide an encouraging sign that a palmitoylethanolamide-containing cream may provide an alternative therapeutic option in the treatment of inflammatory skin conditions.
Mode of Action
The precise mechanism(s) by which palmitoylethanolamide exerts its anti-inflammatory and analgesic effects are not completely understood. It is generally accepted that palmitoylethanolamide's effects are not mediated via classical cannabinoid receptors, a finding that may account for palmitoylethanolamide's lack of psychotropic effects. In contrast, the majority of AEAs actions appear to be mediated via CB1 and/or CB2 receptors in the brain and periphery. One problem with the use of AEA is its psychotropic side effects, which are thought to be mediated by CB1 receptors. However, there is evidence that such unwanted effects may be reduced or eliminated by coadministering low (sub-therapeutic) doses of AEA and another NAE (Calignano et al., 1998; Di Marzo et al., 2001 ; De Petrocellis et al., 2001 ; Lo Verme et al., 2005b). For example, sub-analgesic doses of palmitoylethanolamide or AEA provide analgesia when given in combination at the same low doses (Calignano et al., 2001).
At the molecular and behavioural levels, palmitoylethanolamide interacts with a number of important targets in the body. Evidence gathered from in vitro and in vivo studies indicates that palmitoylethanolamide reduces oedma, mast cell proliferation, neutrophil infiltration and a number of endogenous mediators of inflammation including: mast cell degranulation (preventing release of histamine and serotonin), cyclo-oxygenase-2 (COX-2) activity, endothelial nitric oxide synthase activity, nitric oxide production from macrophages and lipid peroxidation during acute hypoxia (Gulaya et al., 1998). In addition, palmitoylethanolamide reduces hyperalgesia in animal models of inflammatory pain (Jaggar et al., 1998; Farquhar & Smith, 2001; Conti et al., 2002). Recent data suggests that peroxisome proliferator-activated receptor alpha (PPAR-α) is of key importance to the anti-inflammatory actions of palmitoylethanolamide (Lo Verme et al., 2005a).
Source:
Publication number WO2008075978 A2
Publication type Patent Application
Application number PCT/NZ2007/000370
Publication date Jun 26, 2008
Filing date Dec 18, 2007
https://www.google.com/patents/WO2008075978A2?cl=en#legal-events

